NERVE INJURIES
Overview of Neuropathic Pain
Pain is an unpleasant sensory and emotional experience that can have a significant impact on a person’s quality of life, general health, psychological health, and social and economic wellbeing. The International Association for the Study of Pain (IASP 2011) defines neuropathic pain as ‘pain caused by a lesion or disease of the somatosensory nervous system’. Central neuropathic pain is defined as ‘pain caused by a lesion or disease of the central somatosensory nervous system’, and peripheral neuropathic pain is defined as ‘pain caused by a lesion or disease of the peripheral somatosensory nervous system’.
Neuropathic pain is very challenging to manage because of the heterogeneity of its aetiologies,
- Neuropathic Pain
- Neuropathic Pain from mechanisms to treatment
- Etiology and Pharmacology of Neuropathic Pain,
its symptoms and underlying mechanisms (Beniczky et al. 2005). There is often uncertainty regarding the nature and exact location of a lesion or health condition associated with neuropathic pain, particularly in non-specialist settings. Examples of common conditions that have peripheral neuropathic pain as a symptom are painful diabetic neuropathy, post-herpetic neuralgia, trigeminal neuralgia, radicular pain, post-surgical chronic neuropathic pain, and neuropathic cancer pain (such as, chemotherapy-induced neuropathy, neuropathy secondary to tumour antigens, or caused by direct invasion or compression of neural structures). Examples of conditions that can cause central neuropathic pain include stroke, spinal cord injury and multiple sclerosis. Neuropathic pain can be intermittent or constant, and spontaneous or provoked. Typical descriptions of the pain include terms such as shooting, stabbing, like an electric shock, burning, tingling, tight, numb, prickling, itching and a sensation of pins and needles. People may also describe symptoms of allodynia (pain caused by a stimulus that does not normally provoke pain), hyperalgesia (an increased response to a stimulus that is normally painful), anaesthesia dolorosa (pain felt in an anaesthetic [numb] area or region), and sensory gain or loss.
Neuropathic pain in the head face and mouth is becoming increasingly recognised. As this is a reasonably recently recognised entity many clinicians do not recognise these conditions and they often get misdiagnosed as other OFP conditions including Trigeminal neuralgia. This painful condition is always preceded by a traumatic event (surgery, trauma, burn, virus, systemic illness for example demyelination, diabetes, thyroid disease vitamin deficiency). There is usually a demonstrable neuropath of a peripheral sensory nerve.
The neuropathy may be painful, anaesthetic, altered sensation or usually a combination of all three. Please see
TR Lecture Trigeminal nerve injury for patients final in virtual patient day.
There are patient information leaflets available. A pathway for patients with trigeminal neuropathic pain is suggested. Contrary to common belief urgent intervention is required for patients with caused by dental interventions; some have to be treated within 24-30 hours.
Definition and Classifications
International Classification of Orofacial Pain
Trigeminal neuralgia and Post Traumatic Neuropathic Pain have similar pathophysiological features, they are phenotypically very different (Moreau et al., 2017; Thieme, 2016). “International Classification of Orofacial Pain, 1st edition (ICOP)” 2020) defined PTNP as “a unilateral or bilateral facial or oral pain following and caused by trauma to the trigeminal nerve and its branches, which persists or recurs for more than 3 months”. Accidental or intentional iatrogenic damage to the trigeminal nerves, especially the inferior alveolar and lingual nerves during oral surgeries can cause PTNP (Peñarrocha, Peñarrocha, Bagán, & Peñarrocha, 2012; Valmaseda-Castellón, Berini-Aytés, & Gay-Escoda, 2000). Furthermore, the nerve injury can be provoked by oral surgical procedures, such as extraction of impacted lower third molars, local anaesthesia (LA), root canal treatment, placement of a dental implant, fixation of facial fracture and bone grafting (Devine, Hirani, Durham, Nixdorf, & Renton, 2018).
There are several types of trigeminal neuropathic pain which are;
- Post traumatic neuropathic pain (PTNP) -which this section is dedicated to
- PTNP
- Probable PTNP
- Trigeminal neuropathic pain attributed to another disorder
- Trigeminal neuralgia (TN)
- Classic TN with neurovascular compromise identified on MRI
- Secondary TN due to Multiple sclerosis or other identified causes
- Idiopathic TN with Neither NVC or identifiable cause
- Post Herpetic neuralgia
The diagnostic criteria for these conditions are presented by ICOP
International Classification of Orofacial Pain
The Diagnostic criteria for post traumatic trigeminal sensory neuropathy
Post-traumatic trigeminal neuropathic pain
Previously used terms: Anaesthesia dolorosa; painful post-traumatic trigeminal neuropathy.
Description: Unilateral or bilateral facial or oral pain following and caused by trauma to the trigeminal nerve(s), with other symptoms and/or clinical signs of trigeminal nerve dysfunction, and persisting or recurring for more than 3 months.
Diagnostic criteria:
A. Pain, in a neuroanatomically plausible area within the distribution(s) of one or both trigeminal nerve(s), persisting or recurring for >3 months and fulfilling criteria C and D
B. Both of the following:
- history of a mechanical, thermal, radiation or chemical injury to the peripheral trigeminal nerve(s)
- diagnostic test confirmation1 of a lesion of the peripheral trigeminal nerve(s) explaining the pain
C. Onset within 6 months after the injury
D. Associated with somatosensory symptoms and/or signs4 in the same neuroanatomically plausible distribution
E. Not better accounted for by another ICOP or ICHD-3 diagnosis.
Notes:
- Tests that confirm a relevant lesion or disease affecting the trigeminal nerve may, for example, be surgical or radiological confirmation of nerve compression or lesion, nerve conduction study, laser-evoked potentials, blink reflex or skin biopsy confirmation of reduced nerve fibre terminals. Positive findings in these investigations may provide important diagnostic hints at the source of pain. However, all clinical and diagnostic aspects of the pain need to be considered.
- The severity of nerve injuries may range from mild to severe. They include external trauma and iatrogenic injuries from dental treatments such as local anaesthetic injections, root canal therapies, extractions, oral surgery, dental implants, orthognathic surgery and other invasive procedures.
- Specifically following radiation-induced postganglionic injury, neuropathic pain may appear after >3 months.
- Somatosensory symptoms or signs may be negative (e.g. hypaesthesia and/or hypalgesia) and/or positive (e.g. hyperalgesia and/or allodynia). Note that positive somatosensory signs are not specific to neuropathy. Negative or positive somatosensory signs consistent with the distribution of the pain may be sufficient to indicate the presence of a lesion of the trigeminal nerve. The clinical examination is supplemented by laboratory tests such as quantitative sensory testing.
Comments:
The structure and content of the diagnostic criteria for Post-traumatic trigeminal neuropathic pain deviate somewhat from those of Painful post-traumatic trigeminal neuropathy in ICHD-3 in order to comply with IASP criteria. Pain duration ranges widely from paroxysmal to constant, and may be mixed. There may seem to be a partial overlap with Persistent idiopathic dentoalveolar pain with somatosensory changes, but in this condition there may be no clear temporal relationship and the somatosensory changes may not be limited to a neuroanatomically confined area, in contrast to the criteria for Post-traumatic trigeminal neuropathic pain. Neuroablative procedures for trigeminal neuralgia, aimed at the trigeminal ganglion or nerve root, may result in neuropathic pain involving one or more trigeminal divisions and should be coded as Post-traumatic trigeminal neuropathic pain. Such pain may, in some cases, coexist with Trigeminal neuralgia; for example, when the latter recurs following remission. Post-traumatic trigeminal neuropathic pain rarely, if ever, crosses the midline but, over time, it may in some cases become more diffusely distributed.
Probable post-traumatic trigeminal neuropathic pain
Diagnostic criterion:
A. Pain fulfilling all but criterion B2 for Post-traumatic trigeminal neuropathic pain.
Trigeminal neuropathic pain attributed to other disorder
Description: Unilateral or bilateral facial or oral pain in the distribution(s) of one or more branches of the trigeminal nerve, caused by a disorder other than those described above, persisting or recurring for more than 3 months and accompanied by other symptoms and/or clinical signs of nerve dysfunction.
Diagnostic criteria:
A. Pain, in a neuroanatomically plausible area within the distribution(s) of one or both trigeminal nerve(s), persisting or recurring for >3 months and fulfilling criteria C and D
B. A disorder other than those identified in the sections above, but known to be capable of causing, and explaining, the trigeminal neuropathic pain, has been diagnosed
C. Pain has developed after onset of the presumed causative disorder, or has led to its discovery
D. Pain is associated with somatosensory symptoms and/or signs1 in the same neuroanatomically plausible distribution
E. Not better accounted for by another ICOP or ICHD-3 diagnosis.
Note:
- Somatosensory symptoms or signs may be negative (e.g. hypaesthesia and/or hypalgesia) and/or positive (e.g. hyperalgesia and/or allodynia).
Comments:
Trigeminal neuropathic pain may develop secondary to multiple sclerosis, space-occupying lesion or systemic disease, with only the clinical characteristics (quality of spontaneous pain, evoked pain and presence of sensory deficits) distinguishing between Secondary trigeminal neuralgia and Other trigeminal neuropathic pain. Other trigeminal neuropathic pain caused by a connective tissue disease or hereditary disorder is usually bilateral, however, may begin asymmetrically and occasionally present with paroxysmal pain superimposed on the background pain. Patients will eventually develop bilateral sensory deficits and continuous pain, which clarify the diagnosis. MRI is normal, but trigeminal reflexes are invariably delayed or absent.
Chronic post-surgical pain (includes PTNP)
A review of the epidemiology of chronic pain found that there is still no accurate estimate available for the population prevalence of neuropathic pain (Smith et al. 2012). For example, the prevalence of neuropathic pain overall has been estimated to be between 6% and 8%, from postal surveys in France (Bouhassira 2008) and the UK (Torrance 2006). However, these estimates came from studies using different questionnaires. Other condition-specific studies have also mirrored the heterogeneous nature of neuropathic pain. For example, painful diabetic neuropathy is estimated to affect between 16% and 26% of people with diabetes (Jensen et al. 2006; Ziegler 2008). Prevalence estimates for post herpetic neuralgia range from 8% to 19% of people with herpes zoster when defined as pain at 1 month after rash onset, and 8% when defined as pain at 3 months after rash onset (Schmader 2002).
The development of chronic pain after surgery is also fairly common, with estimates of prevalence ranging from 10% to 50% after many common operations (Shipton 2008). This pain is severe in between 2% and 10% of this subgroup of patients, and many of the clinical features closely resemble those of neuropathic pain (Jung et al. 2004; Mikkelsen et al. 2004; Kehlet et al. 2006). Furthermore, a study of 362,693 computerised records in primary care from the Netherlands estimated the annual incidence of neuropathic pain in the general population to be almost 1% (Dieleman et al. 2008). This considerable variability in estimates of the prevalence and incidence of neuropathic pain and similar conditions from general population studies is likely to be because of differences in the definitions of neuropathic pain, methods of assessment and patient selection (Smith and Torrance 2010, Smith et al. 2012). A continuum of neuropathic mechanisms probably occurs across PPTTN and at least some of the dysfunctional orofacial pain conditions. In view of the clinical presentation, the neuropathic outcome may depend on the balance between the extent of the nerve injury and the influence of genetic and hormonal factors reflecting the psychosocial impact of chronic or/and intense acute stress.
Surgical causes of PTNP
- Third molar extraction (Lingual nerve and inferior alveolar nerve) · Prolonged surgery duration · Patient age · Inexperienced surgeon · Depth of impacted mandibular third molar · Lingual access surgery · The proximity to the inferior alveolar nerve canal. Prevention of these nerve injuries is possible by preoperative risk assessment (Link to lecture
- Prevention and management of nerve injuries
- Mandibular Third Molar
- LA · Block anaesthesia · Multiple block injections · Inferior alveolar nerve · Lingual nerve · Serious pain on injection · The concentration of LA · Type of the LA
- Trials and Tribulations of local anesthesia)
- Root canal treatment · The proximity of the tooth apex to the inferior dental canal · Sodium hypochlorite leakage from the root into the bone (See restorative nerve injury lecture )
- Placement of dental implants · The proximity of the surgical area to the inferior dental canal · Placement of an implant longer than 10 mm (Seerestorative nerve injury lecture)
The reviews conducted by Renton and Van Der Cruyssen, here and here reported that damage to the trigeminal nerve can be temporary, however, the temporary damage can become permanent over time. The temporary damage is mostly caused by LA or extraction of the third molar, while limited evidence suggests that damage during implant surgery or root canal treatment is permanent. PTNP patients complain of neurosensory disturbances, such as numbness and changed sensation, without pain or with moderate to severe neuropathic pain (De Poortere, Van der Cruyssen, & Politis, 2021; Van der Cruyssen et al., 2020). PTNP is a continuous, paroxysmal, burning, stabbing, pressure, or itching pain within the 5th cranial nerve divisions 2 and 3 (Benoliel, Zadik, Eliav, & Sharav, 2012; Peñarrocha et al., 2012; Renton & Van der Cruyssen, 2020). The pain can last from seconds to minutes (Benoliel et al., 2012). Iatrogenic damage can impair patients’ daily activities including eating, cleaning teeth, talking, shaving, wearing makeup and kissing (Devine et al., 2018; Smith et al., 2013).
Prevalence
Compare to the pain patients experience after other general surgeries, PTNP is a rare condition, affecting 4 to 5% of the population (Van der Cruyssen et al., 2020). Hillerup (2007) reported that after extraction of the third molar, the prevalence of altered sensation is 11.5% temporarily and 0.6% permanently. This study also reported that the incidence of temporary damage to the inferior alveolar and lingual nerve is between 0.15 to 0.54% and permanent damage is 0.0001 to 0.01%. The prevalence of PTNP in a study conducted by Garg et al. (2019) was 1.55% where men were more affected compared to women. However, according to the recent review by Renton and Van Der Cruyssen (2019), the prevalence of PTNP is unknown as not all the incidents that occur in dental practices are reported.
Presentation and Diagnosis
Accurate and early diagnosis of PTNP can avoid the progress of PTNP towards a permanent state. Reviewing the patients’ pain history and symptoms and carrying out clinical examinations are beneficial in the diagnosis of PTNP (Kohli, Katzmann, Benoliel, & Korczeniewska, 2020). However, the diagnosis of PTNP is sometimes challenging as some patients may have no pain, clinical or radiographic defects (Devine, Taylor, & Renton, 2016). Diagnostic tests, such as questionnaires, patients’ self-reports, visual analogue scale, thermal quantitative sensory testing, clinical neurosensory tests or mental nerve blink reflex could be used to detect altered sensation in PTNP patients (Devine et al., 2018).
Resolution of the nerve injury will depend upon many factors primarily the site of damage (if the cell body is damaged there is no likelihood of recovery), duration of injury, cause of nerve injury, which nerve and patient factors – See Paper on prognosis of Trigeminal nerve injuries
The presentation of neuropathic pain in patients can be variable – see Patient Stories
Patients may present with positive and negative signs of neuropathy. Negative signs include hypoaesthesia (reduced sensory function) with numbness (anaesthesia) or reduced sensation. Alternatively hyperaesthesia, or increased sensory function presents with allodynia ( pain on touch , thermal stimuli (particularly cold drafts or fods and drinks) and occasionally taste hypersentivity (pain with chilli, citrus, curry flavours). Qualitative sensory testing using simple tools such as cotton bud, dental probe and blunt callipers can demonstrate the required neuropathic area, negative and positive signs. The use of some of these tests including thermal quantitative sensory testing and clinical neurosensory tests is difficult in dental practices, therefore, the use of instruments such as a cotton swab (to test light touch sensation), a dental probe (to test pinprick sensation) and cool and warm instruments (to test thermal sensation) are more common in dental practices (Kohli et al., 2020). According to the evidence, the diagnostic test should be carried out on injured and uninjured sides and the patients’ response requires to be recorded and reviewed at follow-up appointments (Devine et al., 2018).
An extensive research base has resulted in minimal clinically relevant improvements in management of patients with neuropathic pain. Stratification approach including holistic factors is becoming a recommend approach in assessment of patients with pain and other disorders
- Demographics -age, gender, ethnicity
- Education
- Social status- income, exercise, diet
- Medical health
- Obesity, Diabetes, MS, hypothyroidism, autoimmune conditions, deficiencies (Vitamins B,C and D, Magnesium, zinc and ferritin)
- Psychological status
- Mood disorders- anxiety and depression
- PTSD
- Somatic disorders
- Catastrophising
- Neuroticism- Hypervigilance
- Fear surgery and fear of pain
- Sleep disorders
- Obstructive sleep apnoea
- Other sleep disorders
- Conditioned pain modulation
- Autonomic disorders
- Genetics
Management
Patients with PTNP often do not have knowledge about their treatment options, which can increase stress and anxiousness; therefore, communicating with patients and educating them on the diagnosis and treatment is an essential step in managing patients with PTNP (Kohli et al., 2020). The treatment of PTNP includes pharmacological, surgical, psychological therapies (Zuniga & Renton, 2016).
Managing patients with PTNP is different to most other pain conditions, improvement of PTNP symptoms is difficult, and recurrence is frequent. Therefore, after a nerve injury happens, treatment options should be considered immediately as symptoms of PTNP may be recoverable in the first 3 to 6 months. After this, the symptoms can become permanent, at which point recovery is rare (De Poortere et al., 2021). Furthermore, Baad-Hansen and Benoliel (2017) stated that early treatment of PTNP can decrease the risk of permanent loss of sensation, further nerve damage, and pain. The treatment of PTNP patients should be based on the data collected during the assessment, pain, the level and effect of the nerve injury on patients’ quality of life. Therefore, the risk-benefit ratio of interventions must be considered before treatment (Renton & Van Der Cruyssen, 2019).
Psychological therapies https://www.nhs.uk/mental-health/talking-therapies-medicine-treatments/talking-therapies-and-counselling/nhs-talking-therapies.
Management of patients with neuropathic pain requires a holistic approach. With a stratified approach one can identify factors contributing to reduced pain tolerance for example psychological disorders, sleep disorders, dietary factors etc. The mainstay of managing patients with refractory pain conditions is using psychological therapies (see Virtual patient pain day). Psychological therapies, such as CBT, adjunct to medical and surgical therapies are advantageous. CBT alone has shown to be successful in managing some PTNP patients as they could cope with the pain but not its impact on their life (Abrahamsen, Baad-Hansen, & Svensson, 2008; Renton & Yilmaz, 2012). Additionally, acupuncture, massage therapy and physical therapy can be used to manage post-traumatic neuropathic pain (Macone & Otis, 2018). Although the aim of available PTNP treatments is to reduce pain and psychological impacts in PTNP patients, the result of these treatments can be disappointing for patients and practitioners as permanent neuropathy has been reported in most affected patients (Renton & Yilmaz, 2012; Van der Cruyssen et al., 2020).
Psychological techniques – cognitive behavioural therapy has shown some benefit in the treatment of chronic pain. Studies of chronic pain management suggest that a combination of psychological, pharmacological and physical therapies, tailored to the needs of the individual patient, may be the best approach
Cognitive and behavioural therapies are both forms of psychotherapy (a psychological approach to treatment) and are based on scientific principles that help people change the way they think, feel and behave. They are problem-focused and practical. The term ‘cognitive behavioural therapy’ (CBT) has come to be used to refer to behavioural therapy, cognitive therapy and therapy that combines both of these approaches. The emphasis on the type of therapy used by a therapist can vary depending on the problem being treated. For example, behavioural therapy may be the main emphasis in phobia treatment or obsessive-compulsive disorder (OCD) because avoidance behaviour or compulsive actions are the main problems. For depression the emphasis may be on cognitive therapy.
In 2005 the Government made a commitment to improve the availability of psychological therapies, the preferred method being cognitive behavioural therapy (CBT) for patients, especially in depressive and anxiety disorders. This led to the launch of the Improving Access to Psychological Therapies (IAPT) programme in 2007, the benefits of which are beginning to come to fruition.
Behavioural therapy
This is a treatment approach based on clinically applying theories of behaviour that have been extensively researched over many years. It is thought that certain behaviours are a learned response to particular circumstances and these responses can be modified. Behavioural therapy aims to change harmful and unhelpful behaviours that an individual may have.
Cognitive therapy
This was developed later and focuses on clinically applying research into the role of cognitions in the development of emotional disorders. It looks at how people think about and create meaning about, situations, symptoms and events in their lives and develop beliefs about themselves, others and the world. These ways of thinking (harmful, unhelpful or ‘false’ ideas and thoughts) are seen as triggers for mental and physical health problems. By challenging ways of thinking, cognitive therapy can help to produce more helpful and realistic thought patterns.
Cognitive therapy was developed in the 1960s by Aaron Beck, an American psychiatrist. He felt that his patients were not improving enough through simple analysis and believed that it was their negative thoughts that were holding them back. At around the same time, another therapist, Albert Ellis, was also realising that people’s negative thoughts and irrational thinking could be underpinning mental health problems. He developed a form of cognitive therapy that has come to be known as rational emotive behavioural therapy (REBT).
Subtypes of cognitive therapy
- REBT: this is based on the belief that we all have sets of very rigid and perhaps illogical, beliefs that can make us mentally unhealthy. It teaches the patient to recognise and spot the beliefs that could be causing them harm and to replace them with more logical and flexible ones.
- Cognitive behavioural therapy (CBT): this is another form of cognitive therapy that combines some of the ideas of cognitive therapy with the more analytical approach of psychodynamic psychotherapy. The client and the therapist work together to look at what has hindered changes in the past, in order to understand better how to move forward in the present. It was founded by Dr Anthony Ryle in the 1970s. The therapy sessions explore the patient’s past and childhood and determine why any problems have happened. They will then look at the effectiveness of any current coping mechanisms that the patient may have and will help the patient find ways to improve these. The work is very active. Diagrams and written outlines may be created to help recognise and challenge old patterns and coping mechanisms that do not work well, and provide revised mechanisms. There is a professional organisation known as the Association for Cognitive Analytic Therapy (ACAT) with a wealth of explanation about the therapy on the website (see link under ‘Further reading & references’, below).
- Acceptance and Commitment Therapy (ACT) is a third wave behavioural therapy (along with Dialectical Behaviour Therapy and Mindfulness Based Cognitive Therapy) that uses Mindfulness skills to develop psychological flexibility and help clarify and direct values-guided behaviour. ACT, does not attempt to directly change or stop unwanted thoughts or feelings, but aims to develop a new mindful relationship with those experiences to free a person up to be open to take action that is consistent with their chosen life values. There is increasing evidence for ACT’s use with chronic pain in both a group and individual setting.
Vitamin supplements (always seek advice form your family doctor or physician)
There is evidence that certain vitamins prevent migraines and may improve neuropathic pain in some patients. These are;
- Vitamin B2 Ribolflavin 400 Mgs daily or 100mgs twice daily
- Magnesium 400-500 mgs daily mst supplements are 200mg so take twice daily
- Vitamin D 000 international Units per week
- Co enzyme Q-10 100mg three times daily
- Melatonin may assist in enhancing sleep cycles and help reduce migraine frequency
Medical management
Chaparro, Wiffen, Moore, and Gilron (2012) stated that pharmacological treatments are important in the management of neuropathic pain. Additionally, researchers found and suggested that pharmacological treatment is useful in managing and reducing pain in PTNP patients after a nerve injury caused by dental implant placement (Park, Lee, & Kim, 2010). Opposingly, a study by Haviv, Zadik, Sharav, and Benoliel (2014) reported that only 10% of PTNP patients have shown pain reduction after pharmacological treatment. Chaparro et al. (2012) stated that combination therapy is recommended as it improves the effectiveness and reduces the risk of adverse effects. Furthermore, using topical medications can reduce the severity of post-traumatic trigeminal neuropathic pain and the risk of adverse effects (Heir et al., 2008). The adverse effects include weight gain, dizziness and dry mouth (Haviv et al., 2014).
Systemic medication
Damage to the somatosensory nervous system causes neuropathic pain in PTNP patients (Schlereth, 2020). There is an international consensus optimal medical interventions for patients with Neuropathic pain. Medical guidelines agree that first line medications should be Tricyclic antidepressants that were many decades ago, prescribed for depression in much higher doses, but due to their sdium channel blocking capacities work well for neuropathic pain in low doses, followed by pregabalin and gabapentin, both of which work on GABA receptors reducing pain transmission. Other medications including
- TCAs (i.e., amitriptyline) are suggested as the first-line therapy in patients with neuropathic pain (Finnerup et al., 2015; Szok, Tajti, Nyári, & Vécsei, 2019).
- Gabapentinoids (i.e., pregabalin and gabapentin)
- Serotonin noradrenaline reuptake inhibitors (i.e., duloxetine and venlafaxine)
- Lamotrigine Kohli et al. (2020) stated that antidepressant and antiepileptic medications are the most commonly used medications to manage this condition.
- Weak opioids (e.g., tramadol and tapentadol) Local medication
All medication can cause side effects and man intolerant to patients Table 1 below.
Local medications
There is high level evidence for the therapeutic value of Botulinum Toxin Type A (BTX-A) as a peripheral prophylactic or symptomatic therapeutic nerve block for neuropathic pain (Attal N). There is level A evidence on the use of BTX-A in treating post-herpetic neuralgia and trigeminal neuralgia and level B evidence in treating post-traumatic and painful diabetic neuropathic pain. In addition, BTX-A is relatively safe and effective with reversible effects is recommended for headache (NICE); migraines. Recent high level efficacy is also reported for and neuropathic orofacial pain conditions such as trigeminal neuralgia and post-herpetic neuralgia. Lower level evidence supports use of BtX-A for orofacial musculoskeletal disorders such as myofascial pain or hyperactivity, cervical dystonia and cervicalgia. When injected into the neuropathic painful location, the toxin can be taken up by peripheral terminals of nociceptive afferent nerve fibers, and this action suppresses peripheral and central release of algogenic neurotransmitters such as glutamate or substance P, thus promoting analgesia (Nathan Moreau, Wisam Dieb, Vianney Descroix, Peter Svensson, Malin Ernberg, Yves Boucher Topical Review: Potential Use of Botulinum Toxin in the Management of Painful Post-traumatic Trigeminal Neuropathy. J Oral Facial Pain Headache. 2017 Winter;31(1):7-18.). However, here is extremely limited evidence available with regard treating trigeminal PTNP with Btx-A.
Topical medications
Topical capsaicin 8% has been shown to be efficacious in the treatment of postherpetic neuralgia, painful diabetic peripheral neuropathy, and HIV-neuropathy (Derry et al. Cochrane Database Syst Rev 1:CD007393, 2017). Topical lidocaine has been widely studied and found to reduce pain in patients with postherpetic neuralgia (Knezevic et al. Pain Manag 7:537-58, 2017). Although many other topical analgesics are available, there is limited data to support the efficacy of other agents. Topical analgesics are a relatively benign treatment for chronic pain conditions including neuropathic pain, musculoskeletal, and myofascial pain. There is evidence to support the use of topical NSAIDs, high concentration topical capsaicin, and topical lidocaine for various painful conditions (Jillian Maloney, Scott Pew, Christopher Wie, Ruchir Gupta, John Freeman, Natalie Strand Comprehensive Review of Topical Analgesics for Chronic Pain. Curr Pain Headache Rep . 2021 Feb 3;25(2):7. doi: 10.1007/s11916-020-00923-2.).
- Capsaicin
- Lidocaine patches are second-line therapy in the treatment of this neuropathic pain (Szok et al., 2019).
- CPD oil
Surgery
Evidence remains poor for the efficacy of surgical intervention for trigeminal nerve injuries (Cochrane 2018). In general, it is accepted that neuropathic pain does not respond to surgery and this is often the main drive for the patient seeking care. As specific nerve injuries contradict surgical intervention (Local anaesthesia and chemical nerve injuries). Lingual nerve injury related to third molar surgery using lingual access approach has a high prevalence of temporary nerve injury and therefore the clinician should wait for the 88-90% of injuries to resolve. However, other nerve injuies related to Implant, Endodontics or third molar surgery should be managed swiftly in accordance with recommendations for spinal sensory nerve injuries.
Institution of early medical interventions, Prednisolone, Vitamin B complex and NSAIDS may minimise neural inflammation and prevent further neural damage.
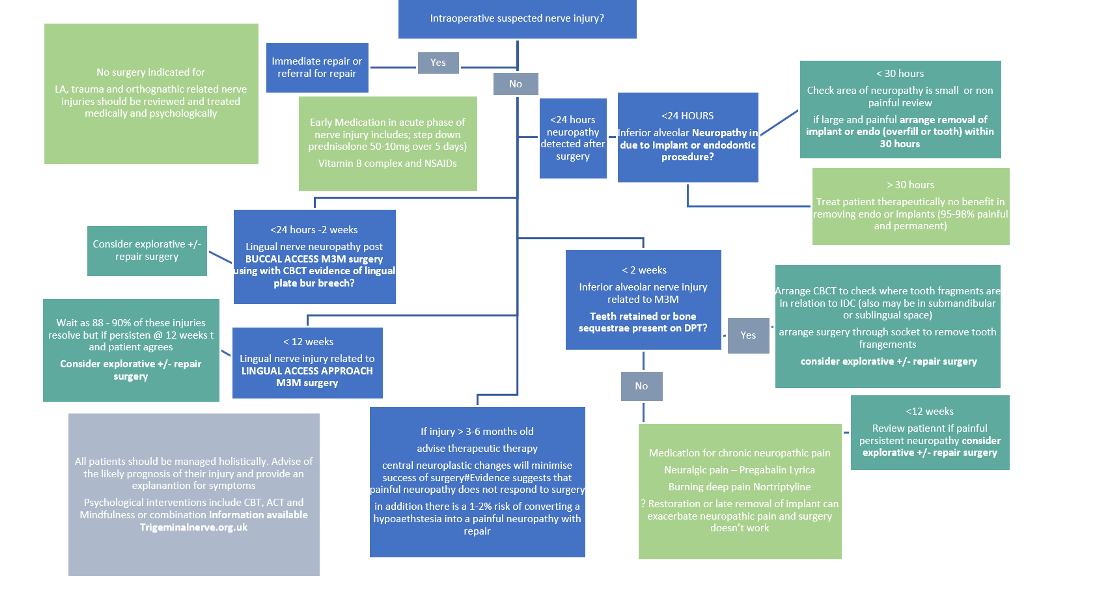
A study conducted by De Poortere et al. (2021) aimed to assess the benefits of surgical therapies in PTNP patients. The researchers observed an improvement in PTNP symptoms and reduction in the use of medication to manage PTNP following surgical therapies. A systematic review conducted by Kushnerev and Yates (2015) reported that surgical therapies should be considered if no improvement has been achieved after the first 3 months following the nerve damage, however, surgery could be delayed up to 6 months if ongoing improvement has been observed. Immediate surgery is sometimes required to resolve nerve injuries. For instance, if the nerve injury is caused by the placement of a dental implant or a root canal treatment, removal of the placed implant or root canal treated tooth should be considered within 30 hours to manage the nerve injury (Renton & Yilmaz, 2012).
Timing of surgical intervention
Immediate within 30 hour removal of Implant or endo is recommended
For third molar related nerve injuries, overall, the evidence is poor and ideally all pts should have immediate repair latest few days with CBCT scanning you can detect breech of the lingual plate and Frederic and I have been involved in developing MR neurrography which may also play a role in early detection ID nerve injuries…. both allowing for earlier intervention. we recommend immediate referral for LNIs and IANS. LNI we do a CBCT to look for lingual plate damage and for IANs we look for any teeth fragments or obvious canal damage and access through the fresh socket within 2 weeks maximum. Bagheri et al of 222 cases suggest 9 months max (Bagheri SC, Meyer RA, Khan HA, Kuhmichel A, Steed MB. Retrospective review of microsurgical repair of 222 lingual nerve injuries. J Oral Maxillofac Surg. 2010 Apr;68(4):715-23. doi: 10.1016/j.joms.2009.09.111. Epub 2009 Dec 29. PMID: 20036042.)
Effectivity of nerve repair depends upon many factors primarily the site of damage (if the cell body is damaged there is no likelihood of recovery), duration of injury, cause of nerve injury, which nerve and patient factors – See Lecture
- age of patient Younger better FSF recovery [Fagin AP, Susarla SM, Donoff RB, Kaban LB, Dodson TB. What factors are associated with functional sensory recovery following lingual nerve repair? J Oral Maxillofac Surg. 2012 Dec;70(12):2907-15. doi: 10.1016/j.joms.2012.03.019. Epub 2012 Jun 12. PMID: 22695009.]
- The younger age of patient and technique (Kang SK, Almansoori AA, Chae YS, Kim B, Kim SM, Lee JH. Factors Affecting Functional Sensory Recovery After Inferior Alveolar Nerve Repair Using the Nerve Sliding Technique. J Oral Maxillofac Surg. 2021 Aug;79(8):1794-1800. doi: 10.1016/j.joms.2021.02.036. Epub 2021 Mar 1. PMID: 33781730.)
- presence of neuropathic pain
It is well accepted that neuropathic pain does not respond to surgery in general and more specifically trigeminal nerve injuries (Zuniga & Renton )
- Zuniga JR, Yates DM. Factors Determining Outcome After Trigeminal Nerve Surgery for Neuropathic Pain. J Oral Maxillofac Surg. 2016 Jul;74(7):1323-9. doi: 10.1016/j.joms.2016.02.005. Epub 2016 Feb 18. PMID: 26970144.
- Zuniga JR, Yates DM, Phillips CL. The presence of neuropathic pain predicts postoperative neuropathic pain following trigeminal nerve repair. J Oral Maxillofac Surg. 2014 Dec;72(12):2422-7. doi: 10.1016/j.joms.2014.08.003. Epub 2014 Aug 11. PMID: 25308410.
- Bagheri SC, Meyer RA, Cho SH, Thoppay J, Khan HA, Steed MB. Microsurgical repair of the inferior alveolar nerve: success rate and factors that adversely affect outcome. J Oral Maxillofac Surg. 2012 Aug;70(8):1978-90. doi: 10.1016/j.joms.2011.08.030. Epub 2011 Dec 16. PMID: 22177818.
- Renton Egbuniwe Nerves and Nerve Injuries Vol 2: Pain, Treatment, Injury, Disease and Future Directions 2015, Pages 469-491
- Renton T Van der Cruyssen F Diagnosis, pathophysiology, management and future issues of trigeminal surgical nerve injuries November 2019 Oral Surgery 13 (4)
- Which nerve responds best? Simon Aitkins recently published a series of LNI patients from Sheffield showing positive results (not explicitly pain relief) in patients undergoing LN repair (Atkins S, Kyriakidou E. Clinical outcomes of lingual nerve repair. Br J Oral Maxillofac Surg. 2021 Jan;59(1):39-45. doi: 10.1016/j.bjoms.2020.07.005. Epub 2020 Aug 13. PMID: 32800402.) Similar to two other studies on lingual nerve (Leung YY, Cheung LK. Longitudinal Treatment Outcomes of Microsurgical Treatment of Neurosensory Deficit after Lower Third Molar Surgery: A Prospective Case Series. PLoS One. 2016 Mar 4;11(3):e0150149. :29 cases presented by my PhD Student Frederic in Leuven
Thus the treatment must be tailored to the patient presenting with neuropathic pain using a combination of the strategies provided here. Personalised medical approach is proving more effective than convention treatments and by using a holistic approach the whole patient and not just their pain is addressed (Link 21)
Side effects of drugs commonly used for NePain control
| Drug | Contraindications | Main Side effects |
| Tricyclic AntidepressantsAmitriptylineNortriptyline10mg nocte raising 10 mg per week up to 40mg nocte-maintenance dose | Cardiac conduction abnormalities, recent cardiac events, narrow-angle glaucoma, elderly patients, epilepsy, bipolar disorder. TCAs may enhance the response to alcohol and the effects of barbiturates and other CNS depressants | dry mouth, constipation, urinary retention, sedation, weight gain |
| SRNIsDuloxetineVenlafaxine | pregnancy | nausea, dry mouth, constipation, dizziness, insomniaheadache, nausea, sweating, sedation, hypertension, seizures |
| SSRIs Selective serotonin reuptake inhibitorsFluoxetine | pregnancy | Side effects: nausea, sedation, decreased libido, sexual dysfunction, headache, weight gain |
| Anti epileptics | ||
| TegretolCarbamazepine | liver disease, acute intermittent porphyria, hyponatremia, a serious blood disorder, marrow depression, taken an MAO inhibitor within the past 14 days8% rashes that may be very serious are more likely to occur in persons with a particular gene called “HLA-B*1502”. This gene occurs almost exclusively in patients with ancestry across broad areas of Asia, including South Asian Indians. Patients with ancestry from these areas should have a blood test by their physician to see if they have the “HLA-B*1502” gene before starting treatment | dizziness, diplopia, nauseaTreatment can result in aplastic anaemia. |
| Pregabalin | renal impairment, diabetes, Congestive heart failure, suicide ideation | drowsiness, dizziness, fatigue, nausea, sedation, weight gain |
| Gabapentin | renal impairment, elderly, suicide ideation | drowsiness, dizziness, fatigue, nausea, sedation, weight gain |
| Lamotrigine | avoid abrupt withdrawal, renal impairment, hepatic impairment, suicide risk, pregnancy 1st trimester | dizziness, constipation, nausea; rarely, life-threatening rashes |
Side effects of drugs commonly used for NePain control
TCA= tricyclic antidepressants, GP = Gabapentin, PGB Pregabalin Dulox= Duloxetine
Suggested Mechanisms of Action for Antidepressants and Antiepileptic Drugs Used to Treat Chronic Pain
| Mechanism of action | Drugs |
| Inhibition of norepinephrine reuptake | Tricyclic antidepressants (secondary amines): desipramine (Norpramin), nortriptyline (Pamelor) |
| Inhibition of norepinephrine and serotonin reuptake | Tricyclic antidepressants (tertiary amines): amitriptyline (Elavil), imipramine (Tofranil) |
| Novel antidepressants: venlafaxine (Effexor), duloxetine (Cymbalta) | |
| Cyclobenzaprine (Flexeril) | |
| Blockade of sodium channel | Antiepileptic drugs: carbamazepine (Tegretol), gabapentin (Neurontin), lamotrigine (Lamictal) |
| Blockade of calcium channel | Antiepileptic drugs: gabapentin, pregabalin (Lyrica)* |
| Enhancement of γ-aminobutyric acid | Antiepileptic drug: carbamazepine |
| Spasmolytic drug: baclofen (Lioresal) |
*—Investigational drug (approval pending from U.S. Food and Drug Administration

