NERVE INJURIES
Neuropathic pain: definition, diagnosis classification and assessment
General pain PAIN, PAIN, PAIN!!!
Pain is a primordial response. Without pain as a protective mechanism, humans may be exposed to harms and dangers that could ultimately threaten the existence of our species! However, pain is distressing and if severe, can be debilitating. Pain can be physical, psychological or both in origin.
Ultimately all physical pains, as perceived by our brains, are mediated by nerves.
A distinction is made between two major types of physical pains due to their differing clinical behaviours. Neuropathic pain is pain due to irritation of the nerves themselves. It is distinguished from the common type of pain (nociceptive pain) that is caused by a cut, burn or injury and nociplastic pain related to chronic widespread pain not linked to specific trauma onset.
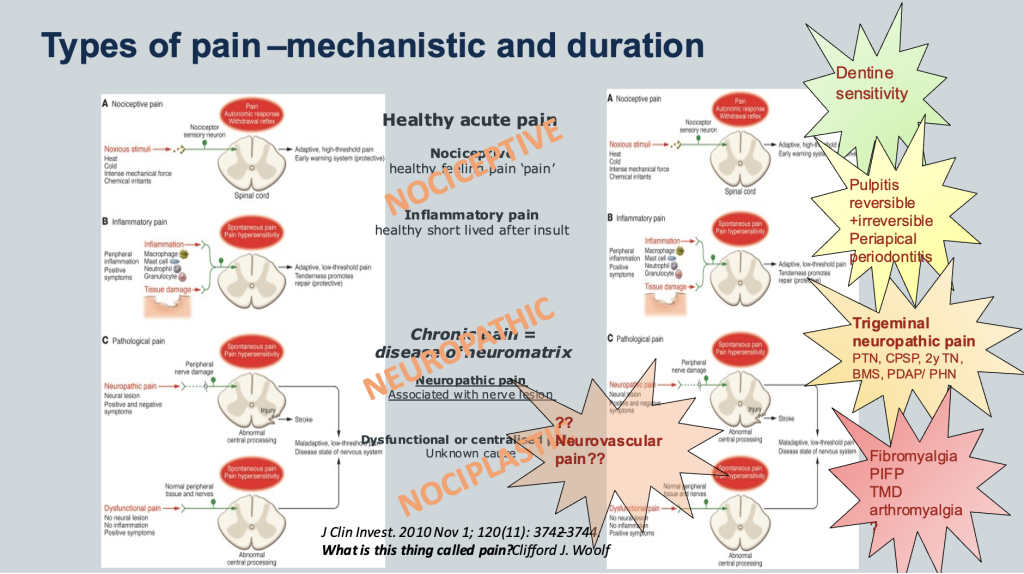
Definitions
Neuropathic pain (IASP) is a Pain caused by a lesion or disease of the somatosensory nervous system.
Neuropathy (IASP) A disturbance of function or pathological change in a nerve: in one nerve, mononeuropathy; in several nerves, mononeuropathy multiplex; if diffuse and bilateral, polyneuropathy. Malfunction of sensory or motor nerves can be caused by many causes; diabetes, demyelination (Multiple Sclerosis), heavy metal poisoning, dietary deficiencies (Vit B complex, Vt D, Magnesium, Zinc), autoimmune diseases, thyroid deficiency and cancer.
Sensor neuropathy may be painless or painful.
Neuralgia – nerve pain
Neuritis (q.v.) is a special case of neuropathy and is now reserved for inflammatory processes affecting nerves.
Classification Chronic neuropathic pain is a major factor contributing to the global burden of disease. Its prevalence ranges between 6.9% and 10% of the general population. How strongly pain is associated with a particular neurological disease varies. Pain may be the most prominent or sole disease manifestation as, e.g., in postherpetic neuralgia, or it may occur only in a subgroup of patients with the same disease as, e.g., in chemotherapy-induced peripheral neuropathies. Even among patients with the same underlying cause of neuropathic pain, painful symptoms and signs usually differ. However, when present, neuropathic pain frequently causes major suffering and disability.
Figure below summarises the IASP classification of neuropathic pain -peripheral (Post traumatic, post herpetic, trigeminal neuralgia, poly neuropathy, painful radiculopathy) and central causes.
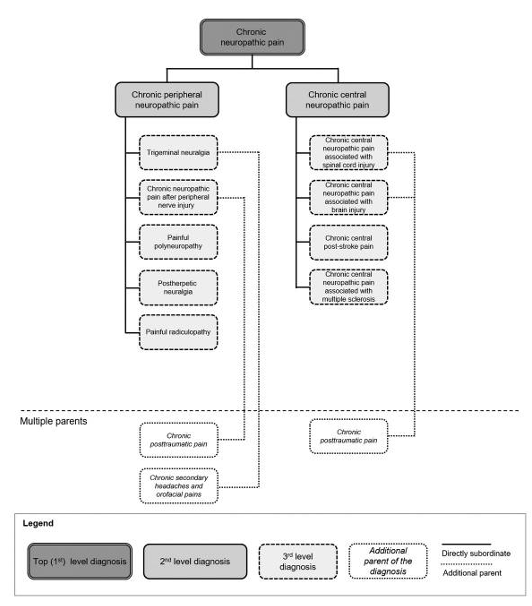
Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, Benoliel R, Cohen M, Cruccu G, Davis KD, Evers S, First M, Giamberardino MA, Hansson P, Kaasa S, Korwisi B, Kosek E, Lavand’homme P, Nicholas M, Nurmikko T, Perrot S, Raja SN, Rice ASC, Rowbotham MC, Schug S, Simpson DM, Smith BH, Svensson P, Vlaeyen JWS, Wang SJ, Barke A, Rief W, Treede RD; Classification Committee of the Neuropathic Pain Special Interest Group (NeuPSIG). The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019 Jan;160(1):53-59. doi: 10.1097/j.pain.0000000000001365. PMID: 30586071; PMCID: PMC6310153. (Click for PDF of paper IASP Nepain)
Post traumatic neuropathy is the term used for sensory nerve injury. We specifically focus on these injuries in the orofacial region which is supplied by the Trigeminal nerve. Trigeminal post traumatic neuropathy is painful in 70% of cases that we see on clinic (90-95% of implant and endo related cases). PTNP is a difficult diagnosis for patients and is difficult to treat. A suspected diagnosis of neuropathic pain requires specific assessment and investigations.
Click for more on PTTN facts and fiction and trigeminal nepain mechanisms
Diagnosis and assessment (PDF Assessment of trigeminal PTNP)
Post-traumatic trigeminal neuropathic pain (PTNP). Neuropathic pain has been recently redefined as pain arising as a direct consequence of any lesion or disease affecting the somatosensory system (Haanpää et al., 2011). Apart from being post-traumatic, the most common diseases related with neuropathic pain are diabetes, HIV, multiple sclerosis and chemotherapy which rarely occur in the head and neck region (Colloca et al., 2017). LINK to COlloca PDF)
Recently, the first edition of the International Classification of Orofacial Pain (ICOP) was published accompanied by its diagnostic criteria.(“International Classification of Orofacial Pain, 1st Edition (ICOP).,” 2020).(LINK to ICOP) Post-traumatic trigeminal neuropathic pain was defined as unilateral or bilateral facial or oral pain caused by trauma to the trigeminal nerve(s), with other symptoms and/or clinical signs of trigeminal nerve dysfunction and persisting or recurring for more than 3 months. The diagnostic criteria are summarized in Table 1.
Table 1. Diagnostic criteria of post-traumatic trigeminal neuropathic pain according to the recent ICOP version 1.
(Previously used terms: anaesthesia dolorosa; painful post-traumatic trigeminal neuropathy.)
| A. Pain, in a neuroanatomically plausible area within the distribution(s) of one or both trigeminal nerve(s), persisting or recurring for >3 months and fulfilling criteria C and D |
| B. Both of the following: |
| 1. history of a mechanical, thermal, radiation or chemical injury to the peripheral trigeminal nerve(s) |
| 2. diagnostic test confirmation of a lesion of the peripheral trigeminal nerve(s) explaining the pain |
| C. Onset within 6 months after the injury |
| D. Associated with somatosensory symptoms and/or signs in the same neuroanatomically plausible distribution |
| E. Not better accounted for by another ICOP or ICHD-3 diagnosis. |
What are the other symptoms of neuropathic pain?
- Allodynia: Pain due to a stimulus, which normally does not provoke pain. For instance, light stroking the skin would cause extremely unpleasant sensation.
- Hyperalgesia: An intensified reaction to a stimulus, which is normally painful. It represents increased sensitivity to pain. For instance, a light tap on a painful area would result in intense pain.
- Paraesthesia: Unpleasant sensations of tingling or pins and needles that occur spontaneously. The area of tingling gives clues to the nerve that is likely involved.
Tests that are considered valid to diagnose PTNP are radiographic or surgical confirmation of nerve injury, nerve conduction study, laser-evoked potentials, blink tests, skin biopsies showing reduced nerve fibre terminals.
However, all clinical and diagnostic aspects should be considered. An important side note: radiation-induced nerve injury may appear beyond 3 months after irradiation but is still considered post-traumatic trigeminal neuropathic pain, hence the large window of onset considered.
Clinical assessment
When assessing patients with surgically induced nerve injuries we recommend a holistic approach (Tara Renton & Van der Cruyssen, 2019). We must treat the patient with the nerve injury, not just focus on the nerve injury itself. Many of these patients have experienced an unexpected traumatic event which demands a thorough history taking and examination including attention for sensory testing and psychological assessment. These elements are necessary both in diagnosis and in the choice of therapy.
The intensity of pain and treatment effect (both in clinic and trials) should be assessed with numerical rating scale or visual analogue scale. For future neuropathic pain trials, pain relief scales, patient and clinician global impression of change and the proportion of responders (50% and 30% pain relief) are important measures. Pain may be reported to be moderate to severe intensity. The pain characteristic is often reported as constant or intermittent burning or elicited and or spontaneous neuralgia. Most cases are continuous, but may report superimposed paroxysmal pain attacks (Benoliel et al., 2012). The pain may be short lasting and neuralgiform elicited due to touch or thermal change usually to cold, in the neuropathic domain, mimicking trigeminal neuralgia (Renton T et al, 2011). Pain is usually unilateral, unless bilateral procedures have successfully injured the patient bilaterally, and may be precisely located to the dermatome of the affected nerve with demonstrable sensory dysfunction
The authors believe that the neuropathy develops immediately after trauma, unless related to endodontic procedure where there may be a 2-3 days delay in neuropathy development. A standardized clinical mechanosensory assessment is illustrated in Figure 2 (source: (Tara Renton & Van der Cruyssen, 2019). These clinical mechanosensory tests have been shown to have a high specificity however they have a low sensitivity (Zuniga et al., 1998). For more accurate sensory profiling, quantitative sensory testing (QST) is recommended for selected cases in clinic, including the diagnosis of small fiber neuropathies and for research purposes (Haanpää et al., 2011). Advanced neurophysiological testing is superior to clinical testing (Teerijoki-Oksa et al., 2019), but this is not always possible intraorally or in large trials involving patient populations. The use of brainstem reflexes and advanced neurophysiologic testing will accurately establish nerve damage (Jääskeläinen, 2004).
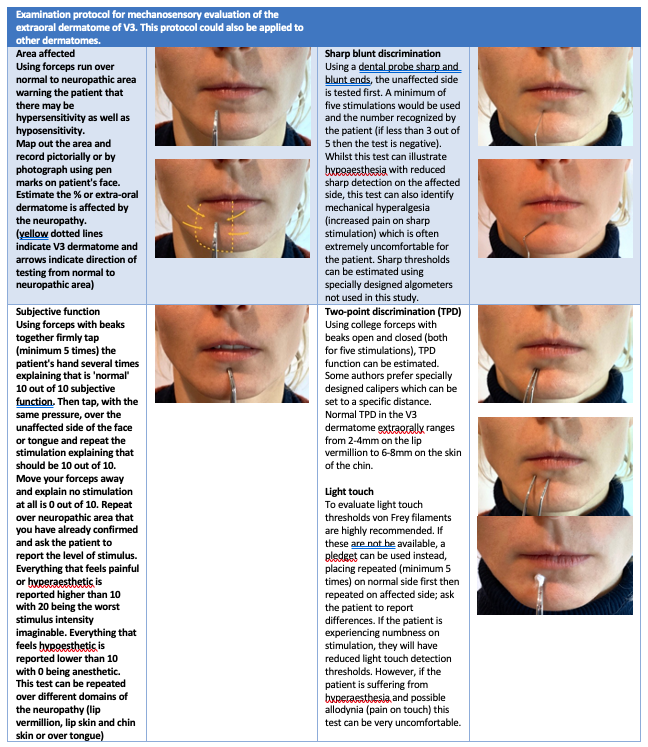
There is almost always an area of abnormal sensation (with the exception in trigeminal neuralgia which is NOT post-traumatic) and the maximum reported pain is associated with the area of sensory deficit (i.e. suffering from a mixture of pain, numbness and altered sensation). This is an important diagnostic feature for sensory nerve neuropathy. Commonly used descriptors are illustrated in Table 2.
Table 2. Commonly used descriptors and their definition (source: IASP).
| Descriptor | Definition |
| Negative symptoms | |
| Anaesthesia | Complete absence of sensation to stimuli |
| Hypoesthesia | Reduced perception of nonpainful stimuli |
| Hypoalgesia | Reduced perception of painful stimuli |
| Thermo hypoesthesia | Reduced perception of heat |
| Positive symptoms | |
| Spontaneous pain | |
| Paraesthesia | Nonpainful tingling sensation |
| Paroxysmal pain | Shooting pain that occurs intermittently for seconds at a time |
| Causalgia | Sustained burning pain, allodynia, hyperpathia can be combined with vasomotor or sudomotor dysfunction and trophic changes |
| Dysesthesia | Unpleasant abnormal sensations, spontaneous or evoked |
| Stimulus-induced pain | |
| Allodynia | Pain induced by a nonpainful stimulus |
| Hyperalgesia | Increased pain by a painful stimulus |
| Hyperpathia – Summation | Increasing amounts of pain due to a repetitive stimulus |
Neuropathic pain commonly presents with allodynia (pain on non-noxious stimuli) hyperalgesia (increased pain to a noxious stimuli) and hyperpathia (continued altered sensation or pain after stimulation ceases). In 50-70% of patients report a combination of numbness, altered sensation and pain is experienced, the pain may be either spontaneous ongoing pain, which often had a burning character, and spontaneous shooting, electric shock-like sensations (neuralgia). Evoked pain due to touch or cold often leads patients to having difficulties with daily function, such as eating, socialising, kissing, speech, and drinking (Fréderic Van der Cruyssen et al., 2020).
Validated neuropathic pain quality measures including Douleur Neuropathique-4, PainDetect and LANSS questionnaires are able to qualify the presence of neuropathic pain but the sensitivity and specificity is reduced in the trigeminal region due to questions around pain wearing clothes and when having a bath for example (Smith et al., 2013).
As a consequence, patients are often anxious, tearful and had psychological repercussions of surgery. These symptoms were often compounded by the lack of informed consent, which was given by only 30% of patients, most of whom were not specifically warned about potential nerve injury (Smith et al., 2013). The presence of anxiety or depression has been suggested to negatively affect treatment outcomes in other pain conditions. In striving for better outcomes, it is therefore advisable to also pay attention to the psychological impact. Psychological assessment requires the use of validated questionnaires exploring anxiety, depression, post-traumatic stress disorder, prior abuse and neglect, sleep quality, catastrophising and somatisation.
Radiological investigations
Routine panoramic radiography is necessary in assessing if after dental extractions the roots remain adjacent to the inferior alveolar nerve and these require removal providing an opportunity to explore and repair the nerve if necessary. Cone beam CT scanning will be required to further assess the relationship between the roots and the mandibular canal which contains the inferior alveolar nerve (Dessouky et al., 2018). Neither medical CTs or MRIs are of great assistance in assessing nerve injuries. However, there are some emerging imaging technologies such as magnetic resonance neurography or multimodal assessments can aid in further diagnostics (Chhabra et al., 2011; Frederic Van der Cruyssen et al., 2020). Radiography after local anaesthetic nerve injuries is of no benefit and both endodontic and implant treatments should be completed with a post-surgical radiograph thus no further radiation is required.

